 永久磁鐵如何運作?(Dr. Don Lincoln, Fermi Lab)
永久磁鐵如何運作?(Dr. Don Lincoln, Fermi Lab)
永久磁鐵的磁性在最近的影片中,我描述了電磁體的工作原理,重點介紹了狹義相對論預測的長度收縮。 這個主題是我最喜歡的主題之一,因為它展示了速度低於每秒一公分的相對論長度收縮。 但你們中的許多人要求的不是電磁體的解釋,而是永久磁鐵的解釋。 這是一個非常有趣的問題。 讓我們深入研究一下。永磁體如何運作的問題當然很有趣。 磁鐵絕對看起來像魔法。 事實證明,很多東西必須排列起來才能製成永久磁鐵。 相關現象涉及基礎粒子物理學、原子物理學和小尺度物理學- - 尺寸為幾分之一毫米。這一切都很複雜,但希望我能說清楚。基礎知識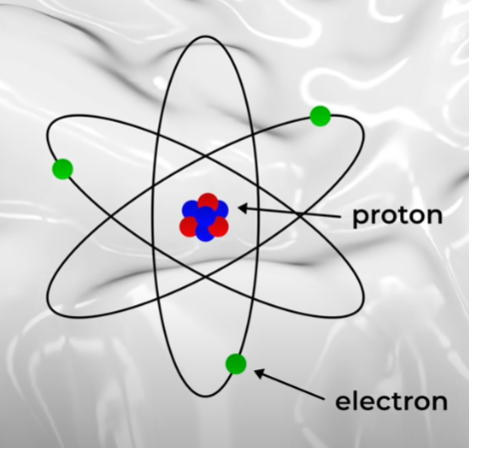 首先是基礎知識。 磁鐵有兩個磁極,北極和南極。 兩者缺一不可。 同極相斥,異極相吸。 兩個指向相反方向的磁鐵其磁性相互抵消,根本不留下任何磁性。 磁鐵是由原子組成的,因此磁性的起源必須來自原子或原子的組成部分(電子與原子核),以及它們的運動方式。原子的相關部分是電子和質子,兩者都帶有電荷並且都可以移動。移動的電荷產生磁場。我製作了一個關於這個的影片——順便一提,這是一個非常酷的影片——所以讓我們看看原子的移動電荷是否發揮了作用。 質子比電子重約 2000 倍,這意味著它們很難移動。所以質子的運動基本上是無關的。但電子呢?他們的動作要大得多。而且,如果電子繞著原子運行就像行星繞著恆星運行一樣,那麼這種圓週運動就會形成磁鐵。但電子不會那樣移動。相反,它們是在原子核周圍的雲層中發現的。它們向各個方向移動,最終的結果是電子的運動不會產生明顯的磁場。質子和電子本身又如何呢?早在 1922 年,在現在被稱為 Stern-Gerlach 實驗的研究中,研究人員就證明了每個電子本身都是一個微小的磁鐵。十年後,奧托·斯特恩領導的團隊證明質子也是磁鐵。 那麼也許這就是磁鐵的組成部分?事實證明,質子磁鐵的強度大約比電子磁鐵小 700 倍,因此我們可以完全忽略質子——至少從磁鐵的角度來看是如此。這就留下了電子。電子是導致磁鐵成為磁鐵的原因嗎?是的。但答案比這複雜的多。首先,是什麼原因導致電子成為磁鐵?簡短的回答是,沒有人知道。就像其他問題一樣。 為什麼電子具有波粒性?為什麼它帶有電荷?為什麼它有這樣的品質?為什麼它像一塊小磁鐵?為什麼人們要在熱狗上塗番茄醬?所有這些都是沒有好的答案的問題。它們只是我們沒有很好解釋的事實。我不知道——也許弗洛伊德弄清楚了整個番茄醬的事情。但對於其餘的東西,就像單一電子的磁鐵強度一樣,這就是宇宙的樣子。要弄清楚其中的原因還得等待未來的發現。那麼電子的固有磁場強度是永久磁鐵的原因嗎?嗯,是的。為什麼?這是因為故事還有更多內容。讓我們進入這個故事的下一章。
首先是基礎知識。 磁鐵有兩個磁極,北極和南極。 兩者缺一不可。 同極相斥,異極相吸。 兩個指向相反方向的磁鐵其磁性相互抵消,根本不留下任何磁性。 磁鐵是由原子組成的,因此磁性的起源必須來自原子或原子的組成部分(電子與原子核),以及它們的運動方式。原子的相關部分是電子和質子,兩者都帶有電荷並且都可以移動。移動的電荷產生磁場。我製作了一個關於這個的影片——順便一提,這是一個非常酷的影片——所以讓我們看看原子的移動電荷是否發揮了作用。 質子比電子重約 2000 倍,這意味著它們很難移動。所以質子的運動基本上是無關的。但電子呢?他們的動作要大得多。而且,如果電子繞著原子運行就像行星繞著恆星運行一樣,那麼這種圓週運動就會形成磁鐵。但電子不會那樣移動。相反,它們是在原子核周圍的雲層中發現的。它們向各個方向移動,最終的結果是電子的運動不會產生明顯的磁場。質子和電子本身又如何呢?早在 1922 年,在現在被稱為 Stern-Gerlach 實驗的研究中,研究人員就證明了每個電子本身都是一個微小的磁鐵。十年後,奧托·斯特恩領導的團隊證明質子也是磁鐵。 那麼也許這就是磁鐵的組成部分?事實證明,質子磁鐵的強度大約比電子磁鐵小 700 倍,因此我們可以完全忽略質子——至少從磁鐵的角度來看是如此。這就留下了電子。電子是導致磁鐵成為磁鐵的原因嗎?是的。但答案比這複雜的多。首先,是什麼原因導致電子成為磁鐵?簡短的回答是,沒有人知道。就像其他問題一樣。 為什麼電子具有波粒性?為什麼它帶有電荷?為什麼它有這樣的品質?為什麼它像一塊小磁鐵?為什麼人們要在熱狗上塗番茄醬?所有這些都是沒有好的答案的問題。它們只是我們沒有很好解釋的事實。我不知道——也許弗洛伊德弄清楚了整個番茄醬的事情。但對於其餘的東西,就像單一電子的磁鐵強度一樣,這就是宇宙的樣子。要弄清楚其中的原因還得等待未來的發現。那麼電子的固有磁場強度是永久磁鐵的原因嗎?嗯,是的。為什麼?這是因為故事還有更多內容。讓我們進入這個故事的下一章。
永久磁鐵的磁性原子、磁矩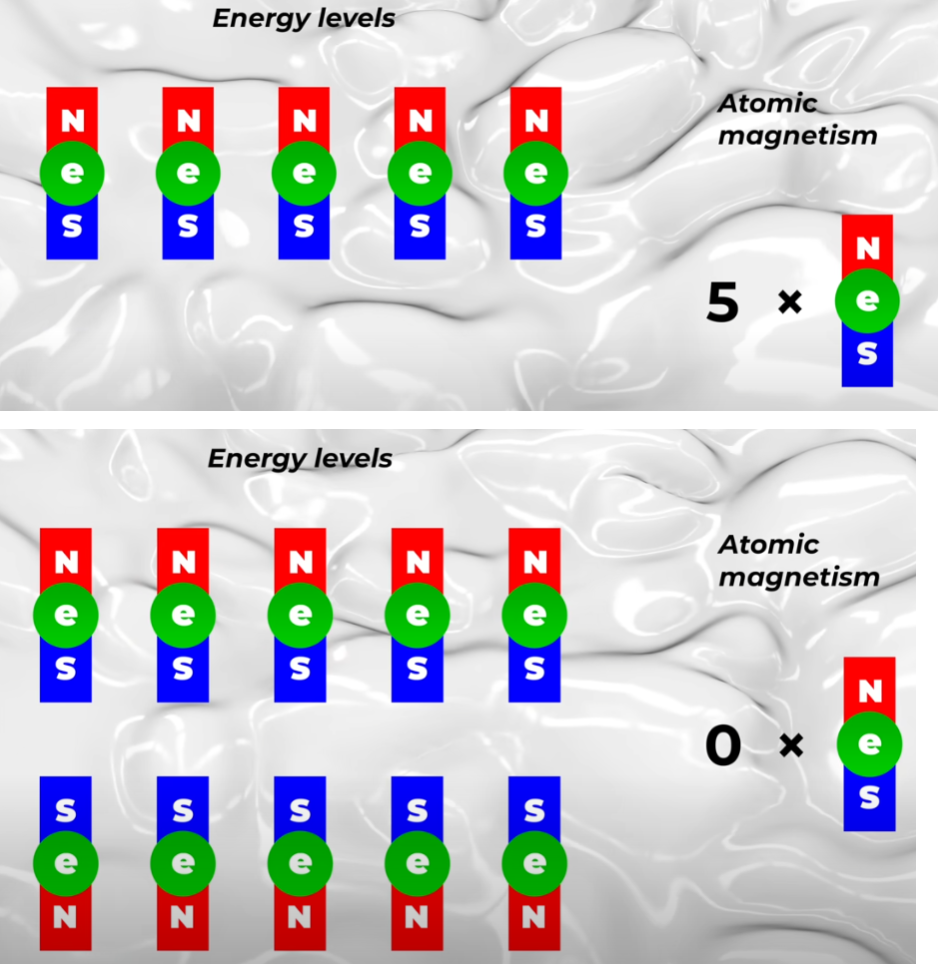 根據薛丁格方程式(當然還有確認測量結果),原子具有一系列重複配置,稱為能階。 每個能階可以接受不同數量的電子 - 2、6、10、14 等。這些能階配置解釋了為何元素週期表具有這樣的形狀。 現在,為什麼所有這些都是真的需要幾個影片來解釋,而我們沒有時間在這裡這樣做。 現在,只要接受這是真的。 量子力學還有另一個與這裡相關的限制,那就是電子如何進入每個能階。 對於一個能階中的一半電子,磁電子可以沿著一個方向定向,但一旦達到中間標記,其餘電子就必須沿著相反方向。 這僅僅是因為量子力學定律以及具有電子自旋的粒子如何聚集在一起。 那麼,讓我們看看這樣做的後果。 我將使用可以包含十個電子的能階之一作為範例。 請記住,為了使原子具有磁性,原子中的電子必須處於同一方向; 否則一個電子就會抵銷另一個電子。 如果我們把一個電子放在能階上,原子就具有一個電子的磁性。 如果我們放入兩個電子,原子就像磁鐵一樣,其強度是單一電子的兩倍。 三個電子,磁性增加三倍。 四四,五五。 但是添加第六個電子,這已經超過了中點,因此電子必須作為與其他電子相反方向的磁鐵進入,因此該原子將僅具有四個電子的磁性。 七個電子意味著原子具有三個電子的磁性。 八意味著二。 九就是一。 十意味著零。 所以這是一個關鍵點。 能量接近空或接近滿的原子不是好的磁鐵。 好的磁鐵是那些充滿了一半軌道的磁鐵。 也就是磁性原子——這讓我們知道了哪些原子是有磁性的,但單個原子的磁性並不大。為了讓磁鐵能夠吸起一個迴紋針,你需要有許多磁性原子指向同一方向。為此,你需要讓許多附近的原子一起工作。
根據薛丁格方程式(當然還有確認測量結果),原子具有一系列重複配置,稱為能階。 每個能階可以接受不同數量的電子 - 2、6、10、14 等。這些能階配置解釋了為何元素週期表具有這樣的形狀。 現在,為什麼所有這些都是真的需要幾個影片來解釋,而我們沒有時間在這裡這樣做。 現在,只要接受這是真的。 量子力學還有另一個與這裡相關的限制,那就是電子如何進入每個能階。 對於一個能階中的一半電子,磁電子可以沿著一個方向定向,但一旦達到中間標記,其餘電子就必須沿著相反方向。 這僅僅是因為量子力學定律以及具有電子自旋的粒子如何聚集在一起。 那麼,讓我們看看這樣做的後果。 我將使用可以包含十個電子的能階之一作為範例。 請記住,為了使原子具有磁性,原子中的電子必須處於同一方向; 否則一個電子就會抵銷另一個電子。 如果我們把一個電子放在能階上,原子就具有一個電子的磁性。 如果我們放入兩個電子,原子就像磁鐵一樣,其強度是單一電子的兩倍。 三個電子,磁性增加三倍。 四四,五五。 但是添加第六個電子,這已經超過了中點,因此電子必須作為與其他電子相反方向的磁鐵進入,因此該原子將僅具有四個電子的磁性。 七個電子意味著原子具有三個電子的磁性。 八意味著二。 九就是一。 十意味著零。 所以這是一個關鍵點。 能量接近空或接近滿的原子不是好的磁鐵。 好的磁鐵是那些充滿了一半軌道的磁鐵。 也就是磁性原子——這讓我們知道了哪些原子是有磁性的,但單個原子的磁性並不大。為了讓磁鐵能夠吸起一個迴紋針,你需要有許多磁性原子指向同一方向。為此,你需要讓許多附近的原子一起工作。
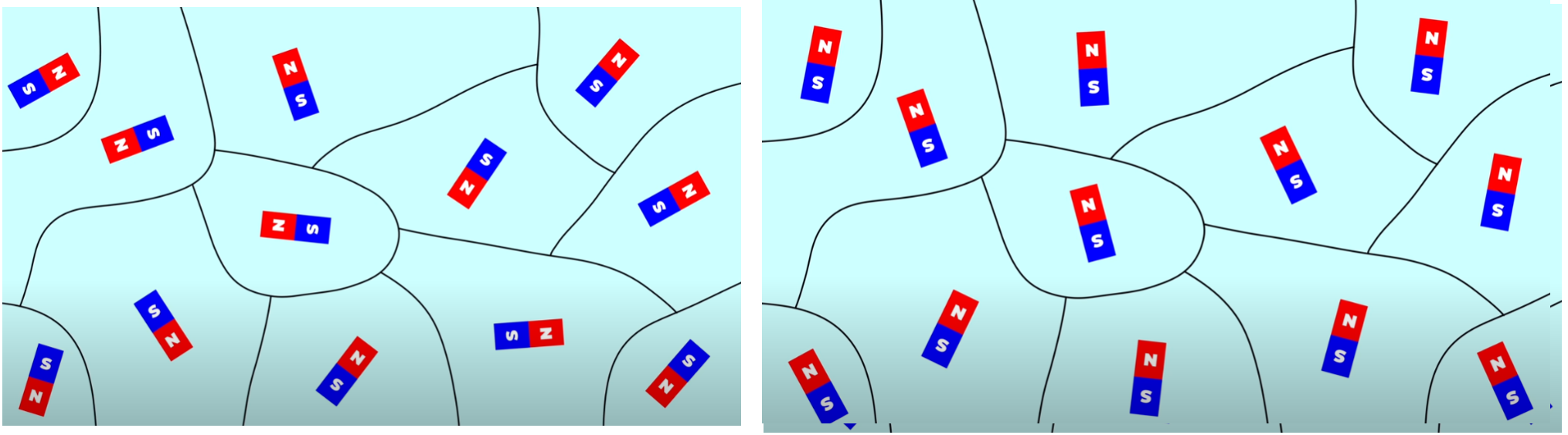
磁鐵的磁矩規則排隊當附近的原子聚集時,在某些情況下它們會形成晶體。 由於原子如何相互作用,這與該元素的特定晶體結構有關,其中一些原子喜歡讓所有原子定向,以便它們的磁性配置對齊。 此類元素稱為鐵磁性元素。 Ferrous 是鐵的拉丁語名稱,這是有道理的,因為傳統的磁鐵是由鐵製成的。 傾向於具有相反構型的相鄰原子的元素被稱為反鐵磁性的。 鐵磁性材料具有潛在磁性,而反鐵磁材料通常不具有磁性。 這是一個很好的地方來指出為什麼原子的磁性並不是故事的全部。 鉻是一種磁性原子,但它不是磁性物質,因為它在晶體水平上具有反鐵磁性。 好的,我們已經介紹了電子、原子和晶體。 是這樣嗎? 不完全的。 還有一個考慮因素。 鐵通常沒有磁性。 我的意思是,鑄鐵煎鍋通常不是。 然而鐵可以變得有磁性。 發生什麼事了? 原因是,即使你有磁性晶體,並且這些晶體影響其他晶體,這種影響也不會永遠持續下去。在大多數鐵中,都有所謂的磁疇。 這些域很小——從用於製作麵包的一粒麵粉大小到罌粟種子大小。 在這些小區域中,晶體結構迫使磁性原子全部對齊。 每個磁域都是磁性的。 然而,附近的其他磁域指向其他方向,最終的結果是,當您將所有磁域的磁性相加時,金屬物體就沒有磁性了。 然而,如果所有磁疇碰巧都朝同一方向取向,則結果就是磁鐵。 那麼如何才能實現這一點呢? 一種方法是取一塊鐵,我們知道它含有許多磁疇,並將其置於強外部磁場中。 這迫使所有磁域指向同一方向,從而將一塊鐵變成磁鐵。 這就是基本的想法。 為了使一塊金屬成為磁鐵,它需要發生四個不同的事情。 首先,電子本身必須是磁鐵。 第二個是電子必須位於大約一半能階被填滿的原子中。 這給了你磁性原子。 第三,晶體結構必須使得原子偏好有小原子磁體全部對齊。 這會為你帶來小的磁疇。 第四,最後,您需要對齊磁疇。如果這四件事都成立,那麼你就擁有一塊磁鐵。 這不是魔法。 這是科學。幾千年來,磁鐵一直讓人著迷。 人們曾經認為它們具有神奇的特性,這是完全可以理解的。 我希望這個影片能讓您對整個主題有一些了解。 如果是,請訂閱該頻道並在社交媒體上分享。 並回來觀看我們的更多影片。 我們談論的是一些瘋狂的東西,但你看的越多,你就越會意識到科學最真實的陳述之一,那就是物理學就是一切。 |
How do magnets work?(Fermi Lab)
In a recent video, I described how electromagnets work, with a focus on the length contraction predicted by special relativity. That topic is one of my very favorites, because it demonstrates relativistic length contraction with velocities under a centimeter per second. But many of you asked for an explanation not of electromagnets, but permanent ones. And that's a very interesting question. Let’s dig into it.(intro music)The question of how permanent magnets work is certainly interesting. Magnets most definitely seem like magic. It turns out that a lot of things have to line up just so to make a permanent magnet. The relevant phenomena involves fundamental particle physics, atomic physics, and small-scale physics- sizes in the ballpark of a fraction of a millimeter. =It’s all very complicated, but hopefully I can make it clear. The basicsSo first the basics. Magnets have two poles, a north and a south. You can’t have one without the other. Like poles repel and opposite poles attract. And two magnets pointing in the opposite direction cancel each other out, leaving no magnet at all. Magnets are made of atoms and therefore, the origins of magnetism must come from the atoms or the constituent of atoms and how they move. The relevant bits of atoms are electrons and protons, both of which have electric charge and both of which can move. Moving charges generate magnetic fields. I made a video about that- it was a way-cool video, by the way- so let’s see if the moving charges of the atom play a role. Protons are about 2,000 times heavier than electrons, which means that they're harder to move. So the motion of protons is basically irrelevant. But what about electrons? Their motion is much greater. And, if an electron orbited an atom like a planet orbits a star, that circular motion would make a magnet. But electrons don’t move that way. Instead, they're found inside clouds surrounding the atomic nucleus. They move in all directions and the net effect is that the motion of electrons doesn’t generate significant magnetic field. What about the protons and electrons themselves? Back in 1922, in what is now called the Stern-Gerlach experiment, researchers proved that each electron is a tiny magnet all by itself. A decade later, a team led by Otto Stern showed that protons are magnets too. So maybe that’s what makes a magnet? It turns out that the strength of a proton magnet is about 700 times less than an electron magnet, so we can ignore the protons entirely- at least from a magnet point of view. That leaves the electron. Are electrons what cause a magnet to be a magnet? Well, yes. But it’s more The short answercomplicated than that. To begin with, what causes an electron to be a magnet? The short answer is, well, nobody knows. It’s like other questions. Why does an electron have a wave and particle nature? Why does it have an electric charge? Why does it have the mass it does? Why does it act like a tiny magnet? Why do people put ketchup on hot dogs? All of those are questions with no good answers. They’re just facts for which we have no good explanation. I dunno- maybe Freud figured out the whole ketchup thing. But for the rest, like the strength of magnet for a single electron, this is just the way the universe is. Figuring out why will have to wait for some future discovery. So is the intrinsic magnetic strength of electrons the cause of permanent magnets?Well, yes. But.Why but? It’s because there is more to the story. Let’s move to the next chapter of this story. According to the Schrodinger Equation (and confirming measurements, of course), atoms have a series of recurring configurations called Energy levelsenergy levels. Each energy level can accept a different number of electrons – 2, 6, 10, 14, etc. These energy level configurations explain why the periodic table of elements has the shape it does. Now just why all of that is true would take several videos to explain and we don’t have time to do that here. For now, just accept that it's true. There is another restriction from quantum mechanics that is relevant here, and that’s how the electrons are allowed to enter each energy level. For half of the electrons in an energy level, the magnetic electrons can be oriented in one direction, but once you’ve hit the halfway mark, the rest of electrons have to be in the opposite direction. This is simply because of the laws of quantum mechanics and how particles with the spin of an electron congregate together. So, let’s see the consequences of that. I’ll use one of the energy levels that can contain ten electrons as an example. Remember that in order for an atom to be magnetic, the electrons in the atom have to be in the same direction; otherwise one electron cancels another. If we put one electron in the energy level, the atom has the magnetic properties of one electron. If we put two electrons in, the atom acts like a magnet twice as strong as a single electron. Three electrons, triple the magnetism. Four- four, and five– five. But add a sixth electron and that’s past the halfway point, so that electron has to enter as a magnet oriented opposite the others, so this atom will only have the magnetic properties of four electrons. Seven electrons means the atom has the magnetism of three electrons. Eight means two. Nine means one. And ten means zero. So this is a crucial point. Atoms with near empty or near full energy levels aren’t good magnets. The good magnets are the ones with half of the orbital filled. Magnetic atomsOkay- that gets us to which atoms are magnetic, but the magnetism of a single atom isn’t much. To have a magnet that will pick up a single paper clip, you need to have lots of magnetic atoms pointing in the same direction. And, for that, you need to have lots of nearby atoms all working together. When nearby atoms assemble, in some cases they form crystals. Because of how the atoms interact, which is related to the specific crystalline structure of that element, some of them prefer to have all of the atoms oriented so their magnetic configurations are aligned. Such elements are called ferromagnetic. Ferrous is the Latin name for iron, which makes sense because traditional magnets are made of iron. Elements that prefer to have adjacent atoms in opposite configurations are called antiferromagnetic. Ferromagnetic materials are potentially magnetic, while the antiferromagnetic ones generally aren’t. And this is a good place to point out why an atom’s magnetism isn’t the whole story. Chromium is a magnetic atom, Magnetic domainsbut it’s not a magnetic substance because it's antiferromagnetic at the crystal level. Okay, we’ve covered the electron, the atom, and the crystal. Is that it? Not quite. There is one more consideration. Iron is not usually magnetic. I mean, a cast iron frying pan generally isn’t. Yet iron can become magnetic. So what’s going on? The reason is that even if you have magnetic crystals and those crystals affect other crystals, the effect doesn’t extend forever. In most iron, what you have what are called magnetic domains. Those domains are small- ranging from about the size a speck of flour used to make bread to about the size of a poppy seed. In these small domains, the crystalline structure forces the magnetic atoms to be all aligned. Each domain is magnetic. However, other nearby domains point in other directions and the net effect is that when you add up the magnetism of all domains, the metal object isn’t magnetic. However, if all of the domains happen to be oriented in the same direction, the result is a magnet. So how can you make that happen? One way is to take a piece of iron, which we know contains many magnetic domains, and put it in a strong external magnetic field. That forces all of the domains to point in the same direction, and this turns a piece of iron into a magnet. So that’s the basic idea. In order for a piece of metal to be a magnet, it needs four distinct things to happen. The first is that electrons have to be magnets all by themselves. The second is that the electrons need to be in atoms who have about half of their energy level filled up. That gives you magnetic atoms. The third is that the crystal structure has to be such that the atoms prefer to have little atomic magnets all aligned. That gives you small magnetic domains. And fourth, and finally, you need to have the domains aligned. If all four things are true, you have a magnet. It’s not magic. It’s science. (phasing sound)Magnets have fascinated people for millennia. It’s completely understandable why people once thought they had magical properties. I hope this video has given you some insight into the whole topic. If it did, please subscribe to the channel and share on social media. And come back and see more of our videos. It's some crazy stuff we talk about, but the more you watch, the more you’ll come to realize one of the truest statements of science, which is that physics is everything.授課教師
陳永忠 ycchen@thu.edu.tw